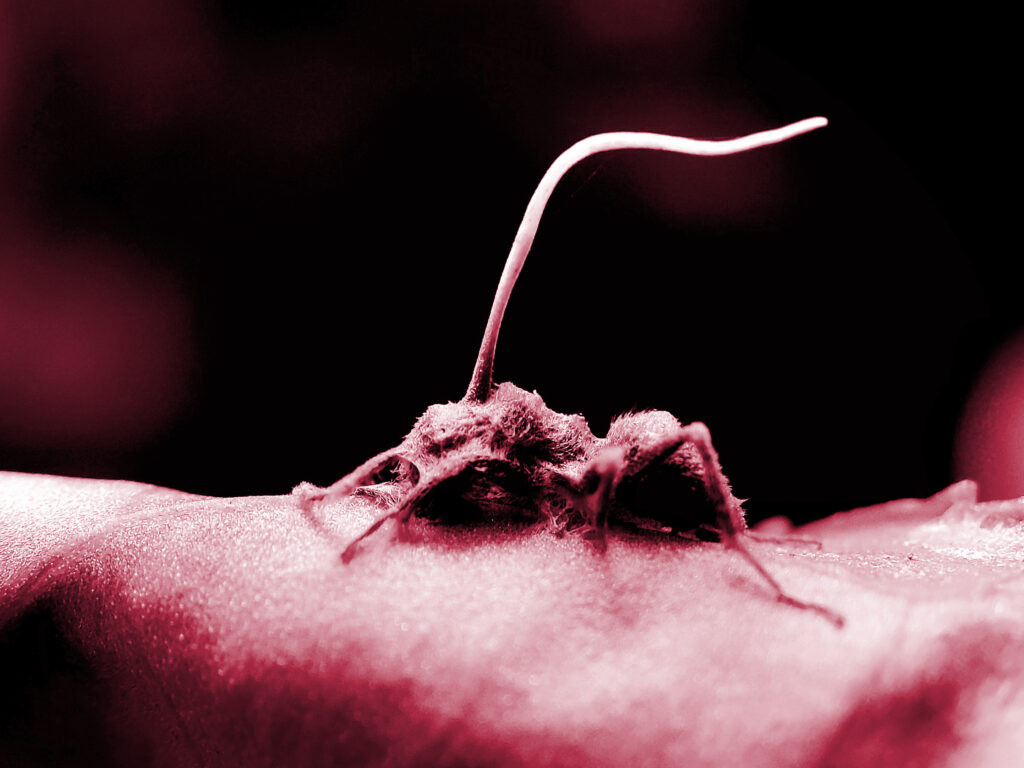
The Last of Us

“The fungus starts to direct the ant’s behavior, telling it where to go, what to do, like a puppeteer with a marionette. And it gets worse,” Dr. Neuman begins.
“The fungus needs food to live, so it begins to devour its host from within, replacing the ant’s flesh with its own. But it doesn’t let its victim die. No, it keeps its puppet alive.”
The other guest, also an epidemiologist, quickly counters, “Fungal infection of this kind is real but not in humans.”
The first scientist concedes, saying it’s true fungi can’t survive in a host with a body temperature above 94 degrees. That would seemingly rule people out.
“But what if that were to change?” he asks.
What if the world began warming, and fungi had a motivation to evolve, with a unifying goal of spreading infection throughout the world? There is nothing to protect us—no vaccines, no treatments, no effective preventive medications—the epidemiologist says.
The studio audience goes quiet. The laughs from the host’s attempts at lightheartedness die.
What happens then, the host of the talk show asks, his voice suddenly unsure.
“We lose.”
A Fungal Zombie Apocalypse?

The opening scene of HBO’s Emmy-winning hit show The Last of Us paints a stark picture of what will come in the video game-based series in which a mutant strain of the Cordyceps fungus evolves to infect humans.
As the first epidemiologist predicted, the fungus has one goal: to proliferate by any means necessary. A pandemic of epic proportions follows, with humans turned into zombie-like creatures hellbent on destruction.
While Cordyceps doesn’t pose much of a threat to people and a fungal zombie apocalypse is a bit far-fetched, the idea of fungi evolving to infect humans is anything but a work of fiction.
“If we change the environment, the soil, the weather, innocuous environmental organisms are going to change,” says Karen Norris, an infectious disease expert, professor in the University of Georgia’s College of Veterinary Medicine, and Georgia Research Alliance Eminent Scholar in Immunology and Translational Biomedicine. “That’s not hypothetical; that’s real.”
More Than Just Mold
When you hear the word fungus, what pops to mind? Mold. Athlete’s foot, perhaps. Or maybe you immediately see a mushroom on a log in a heavily wooded forest.
Probably not a potentially deadly foe.
If we change the environment, the soil, the weather, innocuous environmental organisms are going to change. That’s not hypothetical; that’s real.” — Karen Norris, Georgia Research Alliance Eminent Scholar in Immunology and Translational Biomedicine
But thanks to a changing climate that’s altering soil temperatures, weather patterns, and animal geography, fungi that once didn’t pose a threat to people have become one.
About 6.5 million people worldwide contract invasive fungal infections each year, according to a recent study published in the journal Lancet Infectious Diseases. More than half of those patients die.

The number of fungi-related deaths has doubled to almost 4 million people annually over the past decade.
While rising temperatures are making fungi more adaptable to infect humans, the driving force behind the dramatic increase in fatalities is a far more familiar adversary: drug resistance.
Because many fungi also affect plants and lead to extensive crop losses, anti-fungals known as azoles are regularly used in agricultural fields.
These antifungal treatments are also the first line of defense when people are infected. UGA researchers Marin Brewer, William Terrell Distinguished Professor in the College of Agricultural and Environmental Sciences, and Michelle Momany, a professor in the Franklin College of Arts and Sciences, showed that the compounds used to fight fungal diseases in plants are likely driving resistance to the anti-fungals used to treat people.
But the solution is not as easy as nixing azole use in agriculture.
Each year, fungi destroy about 20% of crops worldwide. And azoles are critical for crop health. Fungicides not only protect plants from harm but can also prevent certain toxins from developing in crops that people consume.
“We need fungicides for food safety and security,” Brewer says. “Fungi evolve incredibly fast. Over the past 20 years or so, we’ve introduced a lot of new fungicides that work really well, but they only work really well for maybe a couple of years.
“Eventually, we might get to the point where nothing works.”
An Arms Race to Antifungal Resistance

The problem is fungi aren’t just developing resistance to one drug. They’re slowly overcoming all of them.
“Fungi are beating us in the arms race to antifungal resistance,” says Emily Rayens MPH ’20, PhD ’21. Rayens is a postdoctoral fellow in epidemiological research at Kaiser Permanente and previously worked as a fellow in Norris’ lab. “The currently available anti-fungal drugs are not doing a good enough job.”
In 2022, the World Health Organization (WHO) published its first-ever list of the 19 most concerning fungal threats to public health.
Topping the list: two types of Candida (auris and albicans), Cryptococcus neoformans, and Aspergillus fumigatus. “A decade ago, nobody had heard of Candida auris,” says Norris. “I certainly hadn’t. Then, suddenly, it started showing up in hospitals and nursing homes.”
Candida auris is a type of yeast that once just lived in the soil. As the Earth began warming, so did the dirt. The fungi adapted, acclimating to temperatures similar to the human body.
The jump into people wasn’t so hard after that.
Candida auris’ journey to becoming a fatal pathogen is a sobering tale, and it’s one that’s becoming much more common.
Many fungi, including the four WHO is most concerned about, are opportunistic creatures. They take advantage of the already weakened immune systems of individuals on chemotherapy for cancer, people living with HIV/AIDS, and those on immunosuppressant drugs.
It used to be that the immunocompromised were the only ones at risk of invasive fungal infections.
But that’s changing.
Norris, Rayens, and José Cordero, Gordhan L. and Virginia B. “Jinx” Patel Distinguished Professor of Public Health, found that the at-risk population has expanded in recent years.
Their study showed that people with diabetes, chronic obstructive pulmonary disease (or COPD), or co-infections such as COVID-19, tuberculosis, or flu are likewise at higher risk of developing fungal infections.
Fighting Back
Fungal diseases are difficult to diagnose and even more difficult to treat.
The epidemiologist in the opening scene of The Last of Us says, “There are no treatments … No preventatives, no cures. They don’t exist. It’s not even possible to make them.”
That’s not quite true.
Health care providers have three classes of drugs to fight fungal infections in people. But many species of fungus are already resistant to one, if not more, of these medications.
But as drug resistance increases, the likelihood that current treatments will no longer work does too.
That’s why Norris is taking a different approach to managing the fungal threat: prevention. There are currently no effective vaccines to protect people from fungal infections. Norris aims to change that.
Her lab developed an experimental vaccine designed to protect against the three most common fungal pathogens responsible for more than 80% of fatal fungal infections.
A recent study tested the vaccine’s efficacy in four preclinical animal models, including nonhuman primates.
The results were promising. The vaccine was effective in developing protective antibodies in each of the models.
Norris hopes to take the vaccine to Phase 1 clinical trials in the coming years. If it performs as well in people as it did in animals, the vaccine could be the first big breakthrough in the fight against one of the top public health threats of this generation.
And it could also prove the epidemiologist from The Last of Us wrong. With an effective pan-fungal vaccine, we don’t lose.